- October 16, 2025
- 0 Comments
- By MelangeMC
Since January 1, 2004, Canada has regulated Natural Health Products (NHPs) under the Natural Health Products Regulations (NHPR), ensuring that products marketed for health benefits are safe, effective, and properly labeled.
Under the NHPR, NHPs include a wide range of products that Canadians use daily to maintain or promote good health:
- Probiotics
- Herbal remedies
- Vitamins and minerals
- Homeopathic medicines
- Traditional medicines (such as Traditional Chinese Medicine)
- Other products like amino acids and essential fatty acids
These regulations fall under the Food and Drugs Act (FDA), and Health Canada oversees their approval, licensing, and marketing.
What the Law Says About Health Claims
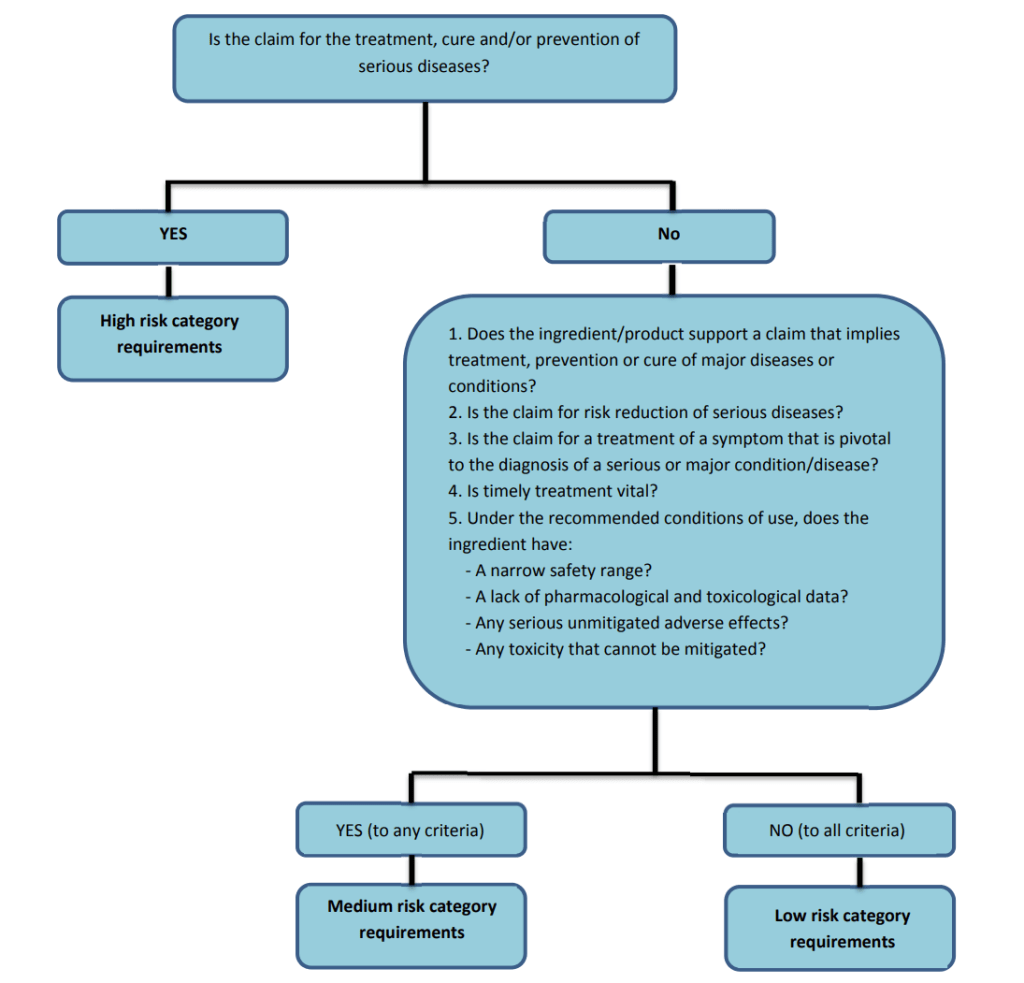
As per section 3 of the FDA and section 103.2 of the NHPR, Natural Health Products cannot be advertised for the treatment, prevention, or cure of diseases listed in Schedule A of the FDA, including on their labels or in promotional materials.
This rule exists because the conditions listed in Schedule A such as cancer, diabetes, asthma, depression, and heart failure, require medical diagnosis and treatment by healthcare professionals. Allowing products to claim they can treat or cure these diseases could mislead consumers and cause harm through self-medication.
Examples of Schedule A Diseases
Below are some of the conditions listed under Schedule A.1 of the Food and Drugs Act, for which NHPs cannot claim to diagnose, treat, prevent, or cure:
- Acute alcoholism
- Acute anxiety state
- Acute infectious respiratory syndromes
- Acute, inflammatory and debilitating arthritis
- Acute psychotic conditions
- Addiction (except nicotine addiction)
- Appendicitis
- Arteriosclerosis
- Asthma
- Cancer
- Congestive heart failure
- Convulsions
- Dementia
- Depression
- Diabetes
- Gangrene
- Glaucoma
- Haematologic bleeding disorders
- Hepatitis
- Hypertension
- Nausea and vomiting of pregnancy
- Obesity
- Rheumatic fever
- Septicemia
- Sexually transmitted diseases
- Strangulated hernia
- Thrombotic and embolic disorders
- Thyroid disease
- Ulcers of the gastro-intestinal tract
A complete and up-to-date list of these diseases is available through Health Canada’s Food and Drugs Act, Schedule A.1.
Why This Matters to Supplement Businesses
For nutraceutical and supplement brands selling in Canada, this regulation is critical. Even if a product contains ingredients that show potential benefits in scientific studies, you cannot claim to treat or cure Schedule A diseases unless the product is classified and approved as a drug under Health Canada’s Food and Drug Regulations.
Violating these advertising restrictions can lead to:
- Product delisting or suspension of your NPN (Natural Product Number)
- Warning letters or enforcement actions from Health Canada
- Loss of consumer trust and reputational damage
Marketing teams should therefore carefully review all labels, packaging, websites, and ads to ensure compliance before going to market.
How to Obtain a License for Natural Health Products and Have Your Claims Approved
Before an NHP can be sold in Canada, it must receive a product licence from Health Canada. This process ensures the product is safe, effective, and of high quality, and that all health claims are supported by evidence.
To obtain a licence, companies must submit a Product Licence Application (PLA) containing detailed information such as:
- Medicinal and non-medicinal ingredients
- Recommended use or purpose (health claim)
- Dose form, strength, and route of administration
- Evidence supporting the safety and efficacy of the claim
Once approved, the product is assigned a Natural Product Number (NPN) or DIN-HM (for homeopathic medicines). This number must appear on the product label to confirm it meets Health Canada’s standards.
For companies seeking to make modern or evidence-based health claims, Health Canada has published a comprehensive guide outlining the licensing process, required documentation, and scientific substantiation expectations.
Here is a direct link to Health Canada’s guidance document:
Pathway for Licensing Natural Health Products — Making Modern Health Claims (v1.0) (PDF)